OMAHA, Nebraska (May 13, 2020)—A new protective device that is expected to help protect healthcare workers everywhere from the novel coronavirus is now available thanks to a remarkable partnership between the University of Nebraska Medical Center and Omaha Custom Manufacturing.
The device has already drawn interest from the U.S. Air Force, which is currently studying the mask’s ability to protect flight crews that transport patients who are infected with COVID-19.
As the Air Force continues its study, the partnership will fast-track the manufacture of the device, called the Infectious Aerosol Capture Mask. It is designed to prevent infected patients from spraying or exhaling viral agents and potentially infecting others in the room.
“Omaha Custom Manufacturing has been incredible to work with,” said Tyler Scherr, PhD, the licensing agent at UNeMed who brokered the deal. “They’re basically doing this for us on a hope and a prayer. They saw the need and are investing to make these devices available to help protect all healthcare workers.”
Added Omaha Custom Manufacturing CEO and President, Mark Keffeler: “If there’s a way for us to help with the pandemic, we want to do it.”
Founded in 1978, Omaha Custom Manufacturing is a family owned, full-service contract manufacturing company that got its start in producing products for use in pharmacies and long-term care facilities—products that are still on the market today.
“Being able to partner with the med center is fantastic, and being able to help with the COVID-19 pandemic adds passion for the project,” said Tyler Keffeler, Vice President at Omaha Custom Manufacturing. Tyler is son to Mark and the third generation of Keffelers in the business.
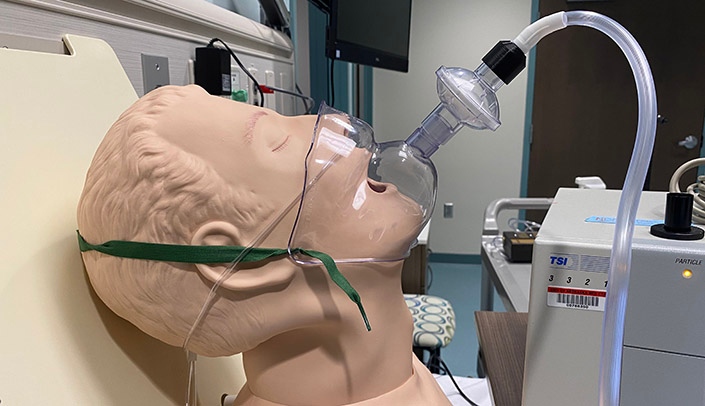
Developed by the chair of UNMC’s Department of Anesthesiology, Steven Lisco, M.D., the Capture Mask is a face tent that covers the patient’s mouth and nose, and is then coupled with a viral filter and a special adaptor that connects the unit to standard vacuum supplies in most clinical settings.
Dr. Lisco—along with director of perioperative imaging, Nicholas Markin, M.D., who 3D printed the adapter—teamed with UNeMed, the technology transfer and commercialization office at UNMC. UNeMed arranged a licensing agreement with a local company, Omaha Custom Manufacturing, which, given the urgency of the pandemic, agreed to focus resources in order to accelerate the device’s speed to market.
It will take about four weeks to set up their manufacturing process for the adapter, about half the time that is customary for injection-molded products. In the meantime, 3D-printed adapters will be sold until the injection molding process is completed, Scherr said.
“This has gone as smooth as possible,” Scherr said, “and the best part is Omaha Custom Manufacturing is right in our backyard. And not only that, but to be a local shop willing to invest their hard-earned money at a time like this, for something like this, is pretty amazing, really.”.
The Nebraska Medicine Innovation Committee has approved the device for use in its facilities, and has already deployed them in operating rooms and elsewhere in the hospital.
Hospitals risk wider contamination from COVID-19 patients when they cough or even just breathe. They produce microscopic particles that float through the air of their rooms, and potentially beyond. Even patients that have no symptoms may still unwittingly spread the virus in the same way, particularly when wearing supplemental oxygen or undergoing the procedures that insert or remove breathing tubes.
Dr. Lisco said in a recent announcement the device performed well in early tests, “catching more than 90 percent of airborne particles expelled in the mask, ultimately preventing the aerosol from entering the patient environment.”
He added: “Even when the vacuum wasn’t turned on, the mask was still 85 percent effective as a barrier.”
At this initial stage, the special adapter for the Infectious Aerosol Capture Mask is available for purchase through Omaha Custom Manufacturing at info@omahacustommfg.com or 800-228-5021. All other components are commonly accessible in most clinical settings and readily found through various medical equipment suppliers.
A future version of the technology will incorporate all components into one contiguous device, but that will not be available for purchase until a later date.