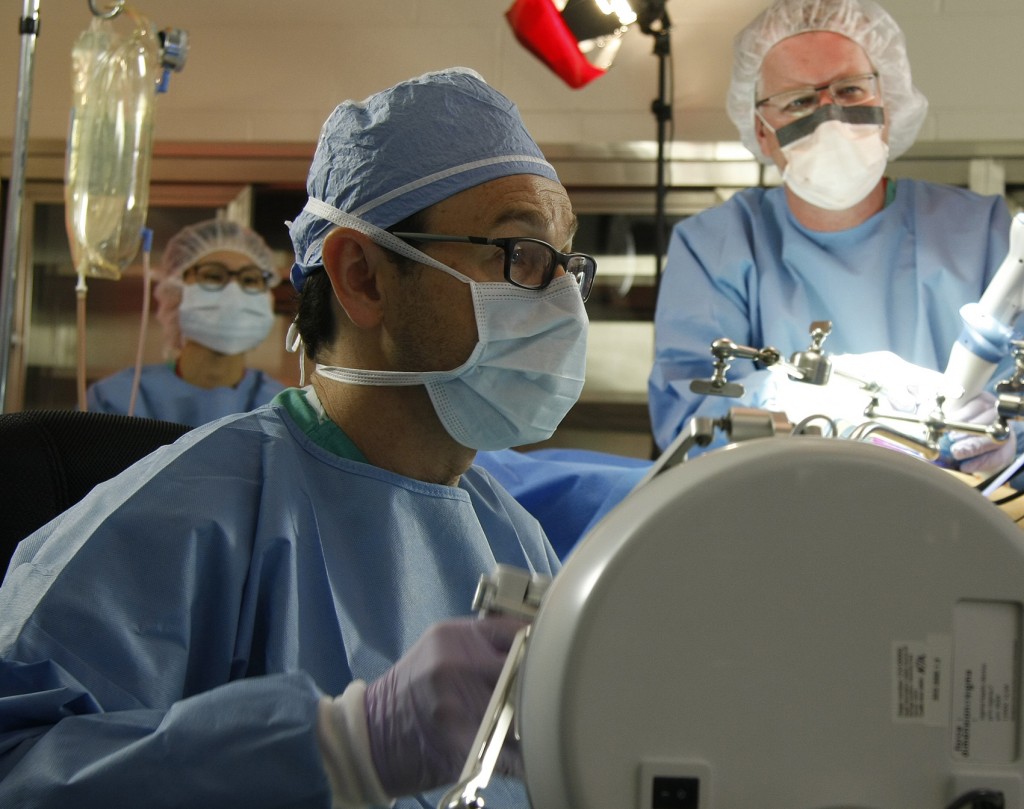
UNMC surgeon Dmitry Oleynikov (left) and UNL engineer Shane Farritor test their surgical robot prototype during a trial several months ago in Omaha. Their collaboration created a startup company, Virtual Incision, which hopes to make major surgery—like a bowel resection—a laparoscopic procedure. (Photo/Charlie Litton)
LINCOLN, Neb. (March 1, 2016)—Virtual Incision Corp., a company founded by faculty at the University of Nebraska-Lincoln and the University of Nebraska Medical Center, has announced the successful first-in-human use of its miniaturized robot-assisted surgical device.
The device is designed for colon resection, a procedure to treat patients with lower gastrointestinal diseases including diverticulitis, pre-cancerous and cancerous lesions of the colon, inflammatory bowel disease and colon polyps that are too large to be removed endoscopically.
“To the best of our knowledge, this is the first time an active miniaturized robot has performed complex surgical tasks with the robot inside a living human, which is a significant milestone in robotics and in surgery,” said Shane Farritor, a UNL professor of mechanical engineering who is Virtual Incision’s co-founder and chief technical officer.
The robot-assisted colon resection procedures were completed in Asunción, Paraguay, as part of the safety and feasibility trial for the technology. The surgeries were successful and the patients are recovering well, according to a news release from the company.
“Virtual Incision’s robot-assisted surgical device achieved proof-of-concept in highly complex abdominal procedures,” said head surgeon Dmitry Oleynikov, chief of minimally invasive surgery at UNMC and co-founder of Virtual Incision.
“Additionally, we verified that our extensive regimen of bench, animal, cadaver, biocompatibility, sterilization, electrical safety, software, human factors and other testing enabled the safe use of this innovative technology.”
Unlike today’s large, mainframe-like robots that reach into the body from outside the patient, Virtual Incision’s robot platform features a small, self-contained surgical device that is inserted through a single midline umbilical incision in the patient’s abdomen. Virtual Incision’s technology is designed to utilize existing tools and techniques familiar to surgeons, and does not require a dedicated operating room or specialized infrastructure. Because of its much smaller size, the robot is expected to be significantly less expensive than existing robotic alternatives for laparoscopic surgery. Virtual Incision’s technology promises to enable a minimally invasive approach to surgeries performed today with a large open incision.
The robot-assisted surgical device is investigational, and not commercially available. John Murphy, Virtual Incision’s CEO, said robot-assisted surgical are beneficial, but existing surgical robots have limitations that prevent pervasive use during certain surgeries, such as colon resection. The firm will build upon the positive completion of the feasibility study, as it works toward clearance for the system in the United States.
More than two million patients undergo colon resection procedures globally each year. About two-thirds of these procedures are performed via a completely open surgical procedure involving an 8- to 12-inch incision and up to six weeks of recovery time. Because the procedure is complicated, existing robot-assisted surgical devices are rarely used for colon resection surgeries, and manual laparoscopic approaches are only used in a third of cases.
In January, Virtual Incision and Nebraska Innovation Campus announced that the firm would be locating its headquarters at the new campus.



 Both bills deal with promoting, supporting and growing start-up and small businesses in the state. For more details of the hearing, see the Lincoln Journal-Star’s coverage
Both bills deal with promoting, supporting and growing start-up and small businesses in the state. For more details of the hearing, see the Lincoln Journal-Star’s coverage  OMAHA, Neb. (January 20, 2016)—Submitting a new invention to UNeMed just got a little easier.
OMAHA, Neb. (January 20, 2016)—Submitting a new invention to UNeMed just got a little easier.
 It was a big year for one of our startups, Virtual Incision, a surgical robotics company built out of a collaboration between UNMC surgeon Dmitry Oleynikov, M.D., and UNL robotics engineering professor Shane Farritor, PhD Virtual Incision is focusing its work on making colon resection surgery—where a piece of damaged or diseased colon is removed—a minimally invasive procedure. In 2015 the company raised more than $11.2 million in the opening round of investing and landed a prestigious robotics
It was a big year for one of our startups, Virtual Incision, a surgical robotics company built out of a collaboration between UNMC surgeon Dmitry Oleynikov, M.D., and UNL robotics engineering professor Shane Farritor, PhD Virtual Incision is focusing its work on making colon resection surgery—where a piece of damaged or diseased colon is removed—a minimally invasive procedure. In 2015 the company raised more than $11.2 million in the opening round of investing and landed a prestigious robotics 
 Our most popular new blog post of the year came from UNeMed’s newest team member, Amanda Hawley, PhD An intern at the time, Dr. Hawley had recently completed her doctorate in cancer biology, and offered advice to fellow scientists looking for alternate career options. Her key recommendation: Challenge yourself with new experiences outside your comfort zone. You might be surprised with what you learn about yourself. Since then Dr. Hawley has been promoted to a full-time postdoctoral position with UNeMed.
Our most popular new blog post of the year came from UNeMed’s newest team member, Amanda Hawley, PhD An intern at the time, Dr. Hawley had recently completed her doctorate in cancer biology, and offered advice to fellow scientists looking for alternate career options. Her key recommendation: Challenge yourself with new experiences outside your comfort zone. You might be surprised with what you learn about yourself. Since then Dr. Hawley has been promoted to a full-time postdoctoral position with UNeMed. Radux, a new company built on a UNMC invention, is the most recent in a long line of startups that has benefited from the University of Nebraska’s Proof of Concept grant funding program. Radux used the cash to build its first working prototypes of devices made to protect physicians from harmful radiation and other injuries that can occur while performing fluoroscopic procedures. Radux was founded by UNMC interventional radiologist Greg Gordon, M.D.
Radux, a new company built on a UNMC invention, is the most recent in a long line of startups that has benefited from the University of Nebraska’s Proof of Concept grant funding program. Radux used the cash to build its first working prototypes of devices made to protect physicians from harmful radiation and other injuries that can occur while performing fluoroscopic procedures. Radux was founded by UNMC interventional radiologist Greg Gordon, M.D.
 Two more UNMC inventors earned national recognition with awards, most recently on Dec. 16 when Rodney Markin, M.D., was named a Fellow of the National Academy of Inventors. “Rod is a rare breed,” said Michael Dixon, PhD, president and CEO of UNeMed. “It’s uncommon to have a skilled clinician who also has such a keen understanding of business and what it takes to develop a product. Not only is he a prolific inventor with 35 patents, but he’s also helped turn those ideas into products – products that have built startup companies or have sold widely in multinational companies.”
Two more UNMC inventors earned national recognition with awards, most recently on Dec. 16 when Rodney Markin, M.D., was named a Fellow of the National Academy of Inventors. “Rod is a rare breed,” said Michael Dixon, PhD, president and CEO of UNeMed. “It’s uncommon to have a skilled clinician who also has such a keen understanding of business and what it takes to develop a product. Not only is he a prolific inventor with 35 patents, but he’s also helped turn those ideas into products – products that have built startup companies or have sold widely in multinational companies.” In May, UNeMed announced that its services now also cover smartphone and tablet applications. UNeMed can help provide or find resources for further development while securing any intellectual property associated with the software. As a registered publisher at iTunes and Google Play, UNeMed can also publish an app to the most common marketplaces.
In May, UNeMed announced that its services now also cover smartphone and tablet applications. UNeMed can help provide or find resources for further development while securing any intellectual property associated with the software. As a registered publisher at iTunes and Google Play, UNeMed can also publish an app to the most common marketplaces.


 One of our most popular posts from 2015 announced the new status of
One of our most popular posts from 2015 announced the new status of 
 Adam Ruben, PhD, from “Outrageous Acts of Science” on the Science Channel, gave a talk during UNMC’s 2015 Innovation Week titled “Public Perception of Science.” He listed examples of the public’s ill-informed response to scientific discovery like cloning animals, vaccines, and the
Adam Ruben, PhD, from “Outrageous Acts of Science” on the Science Channel, gave a talk during UNMC’s 2015 Innovation Week titled “Public Perception of Science.” He listed examples of the public’s ill-informed response to scientific discovery like cloning animals, vaccines, and the 
 by
by  OMAHA, Neb. (November 1, 2015)—Amanda Hawley, PhD, has been promoted from intern to a full-time postdoctoral position as a licensing associate with UNeMed, the technology transfer and commercialization office at the University of Nebraska Medical Center.
OMAHA, Neb. (November 1, 2015)—Amanda Hawley, PhD, has been promoted from intern to a full-time postdoctoral position as a licensing associate with UNeMed, the technology transfer and commercialization office at the University of Nebraska Medical Center.

 “The idea of PortCas is pretty basic,” said Joseph Siu, PhD, who is working with Hanson on the PortCas. “If we can move a humongous, $100,000 simulator, move it into tiny boxes, and make it cheap enough for every medical student in the world to have one, then we’ve met our goal.”
“The idea of PortCas is pretty basic,” said Joseph Siu, PhD, who is working with Hanson on the PortCas. “If we can move a humongous, $100,000 simulator, move it into tiny boxes, and make it cheap enough for every medical student in the world to have one, then we’ve met our goal.” Dr. Al-Murani said the technology is unique because it’s a naturally occurring protein, versatile, easy to manufacture and easy to store and use.
Dr. Al-Murani said the technology is unique because it’s a naturally occurring protein, versatile, easy to manufacture and easy to store and use. Qian Zhang, PhD, of UNeMed presented the Portable Laparoscope, an invention of Chandra Are, M.D.
Qian Zhang, PhD, of UNeMed presented the Portable Laparoscope, an invention of Chandra Are, M.D. Thoraguard is an innovative approach to the chest tubes used to drain fluids. The tubes are prone to clogging, causing Retained Blood Complex, which doubles the mortality rate, increases the length of stay in patients by about a week, and increases the costs to care for a patient by about $30,000. Larger tubes cut down on clogging but bring about a different set of problems, such as increased infections and damage to surrounding organs.
Thoraguard is an innovative approach to the chest tubes used to drain fluids. The tubes are prone to clogging, causing Retained Blood Complex, which doubles the mortality rate, increases the length of stay in patients by about a week, and increases the costs to care for a patient by about $30,000. Larger tubes cut down on clogging but bring about a different set of problems, such as increased infections and damage to surrounding organs. Valeriya Kettelhut, M.D., PhD, presented a system to track infections in hospitals and other high-risk environments, such as transport centers, cancer centers, and long-term facilities.
Valeriya Kettelhut, M.D., PhD, presented a system to track infections in hospitals and other high-risk environments, such as transport centers, cancer centers, and long-term facilities. “Think about how we use chemical today,” said Draper. “Our overuse of chemicals and anti-microbial products are contaminating ourselves, our water, our air, our ground, and creating new contaminants in our environment.”
“Think about how we use chemical today,” said Draper. “Our overuse of chemicals and anti-microbial products are contaminating ourselves, our water, our air, our ground, and creating new contaminants in our environment.”



 by
by