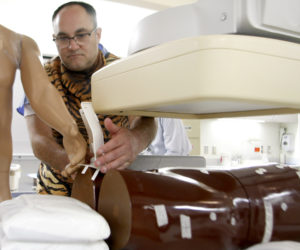
Greg Gordon, M.D., aligns a prototype of his patented Lock-Block radiation shield during a round of early tests. Dr. Gordon built his own startup company, Radux Devices, around inventions designed to better protect physicians from radiation.
OMAHA, Nebraska (Feb. 21, 2017)—Officials at the University of Nebraska Medical Center reviewed their use of the Nebraska Research Initiative’s Proof-of-Concept or POC grant funding program. The review found that despite its relatively modest use, the program has consistently delivered powerful results.
POC grants focus on research innovations and discoveries in danger of slipping into an academic research limbo known as the “Valley of Death.” Innovations that slide into this no-man’s land are usually too advanced to warrant additional funding from various federal agencies, but still too early in development to attract commercial or industrial funding.
The POC grant program at the University of Nebraska was created to fill those gaps for research projects. The program is most often used at UNMC for new biomedical discoveries that have high potential to “improve healthcare for Nebraska and beyond,” said Michael Dixon, the CEO at UNeMed, which performed the review.
“This is one of the few sources of gap funding we have,” Dixon said. UNeMed is the technology transfer and commercialization office for UNMC. UNeMed also works with inventors at the University of Nebraska at Omaha.
“Without POCs,” Dixon added, “we’d have more than a dozen technologies just dying on the vine. That’s a dozen potential treatments, cures and better outcomes that industry just isn’t ready to invest in. But they’re all moving forward now thanks to this program, and several are now receiving financial support from industry. That’s a huge credit to our leaders in the legislature and at the University. They made it happen.”
Over the five-year history of the program, 17 grants totaling $2.78 million have been approved for UNMC and UNO biomedical innovations.
More specifically, the grants led directly to the creation of four startups companies; four new partnerships with biomedical companies; at least $525,000 in additional federal research funding; and another $1.3 million from industrial partners to support of University research. And one UNMC startup company, armed with data produced from a POC-funded study, was able raise an additional $300,000 from outside investors.
POC grants at UNMC and UNO have also led to advanced prototypes of medical devices and drug treatments that move the technologies closer toward their first clinical trials and product launches.
Approval for a POC grant typically requires significant feedback from a potential industrial or commercial partner who is interested in developing the technology.
For example, a company might be interested in a UNMC innovation, if only the researcher could show the result of a particular test. That test result alone could determine whether a new cancer drug or medical device advances closer to market. But with no additional research funds, the innovation will often lie in wait, rarely seen or heard from again.
The POC grants often pay for those additional tests, delivering the results needed to move a technology closer to market.
“The companies interested in these technologies, they just aren’t always in a position to handle the risk of paying for these additional studies,” said UNeMed’s Director of Business Development, Joe Runge. “These tests aren’t just things we pull out of our ears. This is in line with the things that industry needs to see before investing significant time and treasure.”

A new simulator invented at the University of Nebraska Medical Center uses video game technology to teach laparoscopic surgery to residents pressed by work hour restrictions.
Reducing the amount of risk involved for companies is a large part of what makes the program successful, said David Jackson, the University of Nebraska’s Vice Provost who administers the Nebraska Research Initiative and its POC program.
“This is high risk for us too,” Jackson said, “but if someone doesn’t take the risk, then these technologies stay dormant. Just to leave a technology on the table is a lost opportunity.”
He added: “We take that risk because of the potential for moving technologies out the door in a way that’s going to benefit all Nebraskans.”
The NRI was originally created in 1988 as a state-funded program to build research capacity and expertise throughout the University system. Later, in 2011, a small portion of that funding was directed to POC grants, or what Dixon at UNeMed calls “Valley of Death funding.”
About 6 percent of the NRI budget goes to POC funding at all campuses in the University of Nebraska system, Jackson said.
“Looking back, the state deserves a ton of credit for their foresight in setting up the NRI to begin with,” Dixon said. “It helped grow this research engine to the beast it is today. I can’t imagine what this POC program might lead to in another 20 years; what the biomedical sector in Nebraska might look like; all the jobs it might produce. It could be transformative for our local economy, not to mention all the medical benefits we might see.”
At UNMC, all of UNeMed’s approved POC grants resulted in eliminating unknowns and uncertainties for potential industry partners.
“We try to be judicious, and have partners in mind,” Jackson said. “I don’t count it a failure if something like a prototype doesn’t work. That tells you to stop the work, and move in a new direction. What’s a failure in my mind is if something gets funded that doesn’t answer a question industry needs.”
The first POC grant at UNMC, awarded in late 2011, funded a study that created the data necessary to support FDA clearance for a clinical trial. The technology is a unique drug that could allow doctors to diagnose, treat and track tumors in patients who suffer some of the most deadly forms of cancer.
The clear path to a clinical trial helped attract a serial entrepreneur from Kansas City who used the technology to create a startup company, Calidum, Inc. Calidum is now raising funds to perform the trial.
Another POC paid for a small clinical trial related to Parkinson’s disease. The trial yielded such encouraging results that a major pharmaceutical company is interested in repeating the study in a larger group of patients.
Two grants were awarded for UNO innovations created at the Biomechanics Research Building. One innovation led to an advanced prototype of a special insole that fits in a hospital slipper. Sensors within the insole then help predict the risk of falls for at-risk patients.
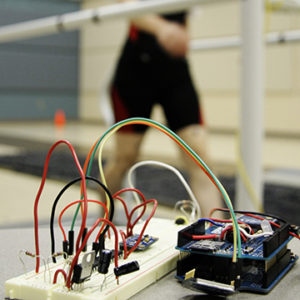
A look at the earliest version of a prototype developed by biomechanics researchers at the University of Nebraska at Omaha for detecting an exacerbation of chronic obstructive pulmonary disease. A POC grant from the Nebraska Research Initiative help create a wearable, advanced prototype that is expected to enter second clinical trial later this year.
The other UNO-related grant paid for a study of chronic obstructive pulmonary disease or COPD. The study produced so much information that researchers had to create a new invention just to interpret the data stream. That simple solution could be applied to several other inventions at UNOs biomechanics facility.
Yet another UNMC innovation led to a million-dollar research collaboration with a major pharmaceutical firm on a long-acting HIV treatment.
Various contractual and confidentiality agreements prevent UNeMed from disclosing company names and some technology details.
Other POC projects include:
- A validation study for Radux Devices, a UNMC startup, proved the effectiveness of two devices that protect physicians from radiation exposure during procedures that require continuous x-ray imaging. Radux plans its first product launch later this year.
- A project that identified an entirely new molecule to target treatment-resistant pancreatic cancer.
- A study for a blood test to predict coronary artery disease.
- A small grant that paid to train UNMC personnel how to operate a new portable simulator for minimally invasive procedures. The training tool is expected to go through its first round of public testing later this year at Creighton University.